This is a case study on Canadian biorisk regulation for Arb Research, in response to a call for proposals by Holden Karnofsky. I’ve spent ~16 hours on this case study. It’s substantially based on Canadian government outputs, and a conversation I had with someone who works at the Canadian Centre for Biosecurity.
Overview of Canadian biorisk regulation
In 2009, Canada passed the Human Pathogens and Toxins Act (HPTA). This came into full effect in 2015 when the Human Pathogens and Toxins Regulations (HPTR) were published.
The Act and Regulations cover biosafety and biosecurity. The main provisions are:
- Licensing: you need a licence to work with listed human pathogens and toxins
- Compliance with the Canadian Biosafety Standard (CBS) is a condition of holding a licence, and the CBS is used to verify compliance.[1]
- The Canadian Biosafety Handbook (CBH) provides guidance on how to meet the requirements of the CBS.[2]
- Compliance with the Canadian Biosafety Standard (CBS) is a condition of holding a licence, and the CBS is used to verify compliance.[1]
- HPTA Security Clearances: you need a security clearance to work with security sensitive human pathogens and toxins
- Reporting requirements: you need to report incidents to the Minister for Health
- Biological safety officers (BSOs): HPTA licence holders need to have a qualified BSO
HPTA is administered by the Centre for Biosecurity, which is part of the Public Health Agency of Canada (PHAC).[3] The Centre is responsible for regulation and enforcement.[4]
PHAC also issues the Canadian Biosafety Guidelines: “a series of biosafety and biosecurity themed guidance documents... that provide further details and recommendations on more specific topics.”[5]
There are currently around 1000 licence holders in Canada.[6]
Most interesting findings
Probabilities are my confidence in the claim (operationalised as the probability that I would still believe the claim after 40 hours’ more work).
- (75%) The system seems relatively comprehensive and flexible, and applies to emerging risks from bioengineered agents as well as existing risks.
- The Human Pathogens and Toxins Act and Regulations apply to the private and the public sector and are legally binding.
- All work with pathogens requires a licence, and work with the most dangerous pathogens also requires a security clearance.
- Reporting incidents is mandatory and is close to real time.
- There’s a database of all biological agents and their regulatory status, ePATHogen. If you want to do research on an agent that isn’t on ePATHogen, you first need to fill in a pathogen risk assessment template, so that the agent can be classified and added.
- Any gain-of-function research must be reported in advance to the licence holder and Biological Safety Officer.
- (85%) Compliance is very high.
- In 2021–22, 98% of compliance issues were successfully responded to within established timelines
- Since the HPTA, there has only been one prosecution, and no licences have been revoked
- It’s possible that high compliance just reflects low reporting, but I think the combination of mandatory reporting, inspections and licence renewals makes this pretty unlikely.
- (60%) Incentives seem more aligned with resolving compliance issues than with avoiding them in the first place.
- The main indicator the Centre uses to track effectiveness is % compliance issues successfully responded to within established timelines, and notification is non-punitive.
- The incentives these things set up have advantages, but they don’t directly create an incentive to minimise the rate of non-compliance in the first place.
- (>99%) The regulations contain provisions for cases of “serious and imminent danger to the health or safety of the public”, which empower the Minister of Health to act more quickly. In these cases:
- The Minister for Health can use an interim order to designate something an Security Sensitive Biological Agent (SSBA).[7]
- The Minister for Health can notify a licence holder orally that their licence is suspended/revoked. The licence holder then has five days to dispose of or transfer any human pathogens or toxins.[8]
- (>99%) There is a list of prohibited pathogens and toxins (currently only containing smallpox).[9] It is illegal to conduct any work with agents on this list.
- (95%) The Ministry of Defence is de facto exempt from some of the regulations.
- The Minister of National Defence can refuse to give relevant information to the Minister of Health/inspectors.[10]
- (99%) The costs and benefits of the regulations were explicitly estimated, and attempts were made to offset the administrative burden.
Notes on the dis/analogy with AI
Points of analogy
- Accident and misuse considerations
- More and less impactful risks to be managed
- There’s foundational research and applications to be regulated
- There are national security implications and interfaces
Points of disanalogy
- The risks in bio are much better understood
- Though AI synthesised pathogens might change this in future
- Less AI research happens in academia and the government than in the case of bio
- The riskiest bio work is more centralised than the riskiest AI work and the government has more influence
- Maybe the intentions of bio researchers are more aligned with the public good?
- I spoke with someone who works at the Centre for Biosecurity. They worked in tobacco regulation before moving to the Centre. In their experience, regulated parties want to be biosafe and want to work compliantly. In tobacco, people were less interested in public health. For those who work with pathogens, the end game is public health rather than economics. The person I spoke with thinks that commercial motivations will be more prevalent in AI.[15]
Scope of the regime
The HPTA covers “controlled activities”: “possessing, handling, using, producing, storing, permitting any person access, transferring, importing, exporting, releasing, abandoning, disposing” of a listed human pathogen or toxin.[16]
The Act covers synthesised and recreated pathogens, as well as cultivated ones.[17]
Canada uses a list-based system. There are various lists, for:
- Toxins[18]
- Human Pathogens, from risk group 2 (moderate) to risk group 4 (high)[19]
- Prohibited human pathogens and toxins (only smallpox at the moment)[20]
- Security Sensitive Biological Agents (SSBAs): the overlap between risk groups 3-4, and the Australia Group list[21]
The Minister for Health can add or subtract something from any of the other lists “by regulation”, following consultation of an advisory committee.[22] The Minister for Health can also use an interim order to designate something an SSBA if there’s “serious and imminent danger to the health or safety of the public”.[23]
There’s a database of all biological agents and their regulatory status, ePATHogen.[24] If you want to do research on an agent that isn’t on ePATHogen, you need to fill in a pathogen risk assessment template, so that the agent can be classified and added.[25]
HPTA does not apply to:
- “a human pathogen or toxin in an environment in which it naturally occurs, as long as it has not been cultivated or intentionally collected or extracted;
- a drug in dosage form whose sale is permitted under the Food and Drugs Act or a human pathogen or toxin contained in such a drug.”[26]
The first exemption means that a lot of clinical work is exempt, but the vast majority of diagnostic facilities are still regulated by the Centre because they work with control samples, and they tend to use the standard in their institutions.[27]
Also, the Minister of National Defence can refuse to give information to the Minister of Health and to HPTA inspectors[28]
Main HPTAR provisions
Licensing
You need a licence to possess, handle, use, produce, store, permit any person access, transfer, import, export, release, abandon, or dispose of a human pathogen or toxin.[29]
Some info:
- No fees[30]
- Different licences for different risk levels of pathogen[31]
- Maximum term is 5 years for least risky pathogens and 1 year for the most risky[32]
- There are conditions, including compliance with CBS[33]
- RG3 and RG4 facilities must submit a biosecurity plan and have a site inspection before licences are issued[34]
- Licence holders need to keep a list of everyone with authorization to access the facility, including visitors[35]
- To conduct scientific research you need to submit a "Plan for Administrative Oversight for Pathogens and Toxins in a Research Setting"[36]
- “The purpose of the Plan is to facilitate the development of internal accountability structures and support accountability structures that currently exist by bridging gaps in the oversight of pathogens at an institutional level.”
- “PHAC has provided the required elements for inclusion and guidance information for each element of a Plan submission to facilitate the compliance with this regulatory requirement.”[37]
- Activity covered by other regulatory regimes
- Inspector/analyst carrying out HPTA functions
- Peace officer or anyone assisting them
- Anyone who collects samples for laboratory analysis of diagnostic testing
- Anyone working under a federal or provincial Act in exigent circumstances
- “A person who carries out laboratory analyses or diagnostic testing with a human pathogen that is neither a prion nor a prescribed human pathogen is exempt from requiring a licence as long as:
- they do not cultivate or otherwise produce a human pathogen; or
- if there is production, it is done using a sealed container that prevents the release of the human pathogen and the sealed container is decontaminated before its disposal or reuse.”
- “A veterinarian who is registered under the laws of a province - and any persons under their supervision - who carry out laboratory analyses or diagnostic testing with a human pathogen that falls into Risk Group 2 are exempt from requiring a licence (Section 7 of the HPTA) on condition that any controlled activities that they conduct in respect to that pathogen are conducted in the course of providing care to animals in a clinical practice in that province.”
If there is “a serious and imminent danger to the health or safety of the public”, the Minister for Health can notify a licence holder orally that their licence is suspended/revoked. The licence holder then has five days to dispose of or transfer any human pathogens or toxins.[38]
HPTA Security Clearance
Any individual who works with or has access to Security Sensitive Biological Agents requires an HPTA Security Clearance.[39]
SSBAs are the subset of risk group 3 and 4 pathogens which are also on the Australia Group list.[40]
You also need an HPTA Security Clearance to work with toxins specified in HPTR above a certain quantity.[41]
Some more info:
- Individuals without a security clearance may access relevant parts of the facility if there is one to one monitoring at all times by someone with security clearance[42]
- Individuals who have had their security clearance suspended or revoked can’t access those parts of the facility under any circumstance[43]
- No fee for security clearance, except $30-150 for fingerprints.[44]
BSOs
Licensed labs must have a biological safety officer.
The qualifications for BSOs are:
- “(a) knowledge of microbiology appropriate to the risks associated with the controlled activities authorized by the licence, attained through a combination of education, training and experience;
- (b) knowledge of the provisions of the Act and the regulations and any applicable federal or provincial legislation; and
- (c) knowledge of the applicable biosafety and biosecurity policies, standards and practices appropriate to the risks associated with the controlled activities authorized by the licence.”[45]
Their functions include reporting to the minister, monitoring compliance, assisting in developing policies, and running training for their institution.[46] They don’t seem to be empowered to shut anything down.
Reporting requirements
HPTA licence holders have a legal obligation to inform the Minister of Health of:[47]
- Inadvertent release of human pathogen/toxin
- Inadvertent production of human pathogen/toxin
- Disease caused by human pathogen/toxin
- Missing human pathogen/toxin
- Any changes to the physical structure, equipment of standard operating procedures of the facility, if they use risk group 3 or 4 pathogens or toxins[48]
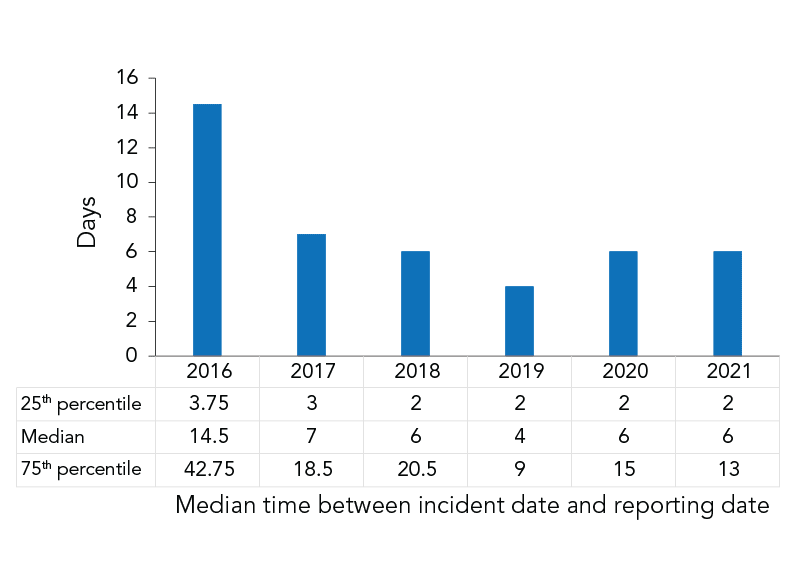
There is an online system for regulated parties to log these incidents.[49] The median time between the incident and the reporting date in 2020 and 2021 was 6 days.[50]
The Centre’s laboratory incident notification group monitors incident reports and triages those which require urgent response. There are internal standard operating procedures which specify which sorts of incident require urgent response.[51]
Notification is non-punitive, and often the first step that the Centre takes in response to a notification is to call up the regulated party and try to understand what happened and why.[52]
Individuals must also notify their licence holder and BSO before carrying out research that increases the virulence, pathogenicity or communicability of an agent.[53]
“Biological safety officers keep an updated list of pathogens and toxins that are in the possession of the institution. PHAC keeps a national inventory of which risk groups are held within each facility. For dangerous pathogens and select toxins the exact inventory and location are recorded. Moderate risk pathogen and toxin inventories are maintained and kept at the institutions and reviewed upon inspection or request.”[54]
There is quite detailed guidance for regulated parties on Notification and Reporting Under the HPTA and HPTR and on Incident Investigation.
Compliance and enforcement
At a high level, the Centre’s main levers on compliance are:
- Training and comms
- Monitoring incidents reported and issuing corrective actions
- Inspection
- Licence renewal
Full compliance activities here.
“Enforcement activities are delineated into 2 categories: Regulatory and Penal Enforcement.”[55]
- Regulatory enforcement
- “It is further divided into administrative and inspection enforcement activities.”
- “The actions may include but are not limited to the following: issuance of letters of non-compliance; refusal to issue the Pathogen and Toxin Licence; variance or suspension or revocation of the Licence; undertake activities related to seizure, detention, forfeiture or disposal; or issuance of orders by an inspector.”
- Penal enforcement
- “Besides its regulatory and inspection mandate, the Centre has a law enforcement mandate. Both the HPTA/HPTR and HAA/HAR have offence provisions.”
- “The Centre's penal enforcement consists of two activities: investigation and prosecution.”
Criminal penalties
Maximum fines range from $50,000 to $1,000,000; maximum sentences range from 3 months to 10 years, depending on the severity of the risk posed. See here for a summary of all penalties.
There are exemptions where you’re not criminally liable if you “exercised all due diligence to prevent the commission of an offence under this Act”.[56] (There are also exemptions to these exemptions.)
Inspection
Certified inspectors can enter any facility “at any reasonable time”,[57] and have extensive powers.[58]
RG3 and RG4 licence holders are inspected once per licence term - every 3 years for RG3, and every year for RG4.[59]
RG2 licence holders are currently inspected every 8 years or so, though the hope is to reduce this to every 5 years, which is the maximum licence term.[60] Apparently RG2 labs constitute a disproportionate number of exposure incidents.[61]
There are currently 20 microbiology inspectors, 4 engineering inspectors, 2 inspection managers, and one engineering manager.
Infrequently, there are targeted document reviews in lieu of inspection, where for example scheduling is difficult.[62]
The Minister of Defence can refuse to provide information.[63]
How effective is the regime?
Probabilities are my confidence in the claim (operationalised as the probability that I would still believe the claim after 40 hours’ more work).
I think that:
- (75%) The Canadian system is quite effective relative to other systems. Reasons I think this, from most to least important to my view:
- (70%) It would be less effective at dealing with very extreme events than median events, and this is problematic. Reasons I think this, from most to least important to my view:
- (60%) Incentives seem more aligned with resolving compliance issues than with avoiding them in the first place
- (95%) The median time between incident date and reporting date is 6 days
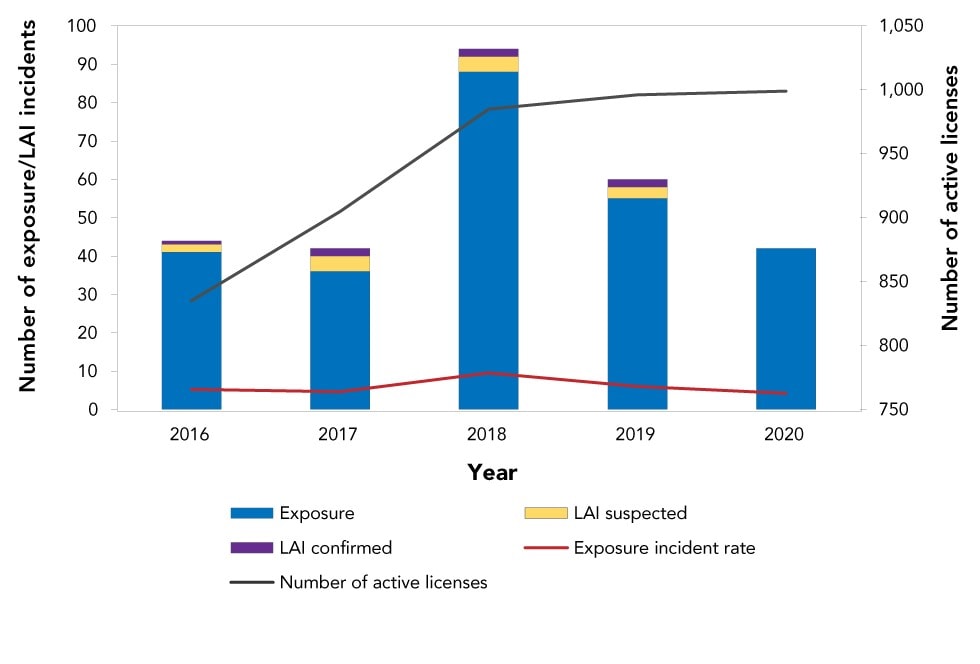
- (95%) ~5% of licence holders have an exposure incident every year
- (90%) At the time of the 2019 audit, there was the potential for COI in case of an incident at the National Microbiology Lab, which was also the only RG4 lab in Canada
- (85%) AI synthesised agents might be hard to manage safely
Strongest arguments for effectiveness
Compliance is very high
- Since 2018, the Centre has met its target of 85% compliance issues in Canadian laboratories successfully responded to within established timelines, which is the main indicator the department uses to track “Public health risks associated with the use of pathogens and toxins are reduced”.[64] This indicator has been at 98% or higher for the past three years.
- Regulated parties are given time to correct deficiencies with standards/non-compliance with HPTA and HPTR
- Someone who works at the Centre for Biosecurty told me that in recent years, in cases where issues were not resolved, this was because they were resolved late, and not because regulated parties were opposing the corrections[65]
- There has only been one prosecution since the HPTA in 2009.[66]
- No licences have been revoked (though licence holders are good at applying for changes in their licence where appropriate)[67]
- In 2014, “71% (181 of 234) of survey respondents indicated that their organization had adopted additional safety practices as a result of their engagement with the Centre for Biosecurity”, and a further 29% “reported that their organization was already in line with the requirements.”[68]
It’s possible that high compliance just reflects low reporting, but I think the combination of mandatory reporting with inspections and licence renewals makes this pretty unlikely.
The Canadian system is well-regarded internationally
- The CBS is used by other countries,[69] and Canada is involved in international efforts to increase biosecurity[70]
- The WHO 2018 Joint External Evaluation rated Canada 5/5 for “Whole-of-government biosafety and biosecurity system is in place for human, animal, and agriculture facilities”, and 4/5 for “Biosafety and biosecurity training and practices”.[71]
- The Global Health Security Index ranks Canada first in the world for biosafety (along with 15 other countries including the US), second for dual-use research (after Armenia, along with 4 other countries including the US), and third for biosecurity (after the US and Denmark).[72]
- Canada has a perfect score on biosafety (100.0), but dropped points on biosecurity (86.7) and dual-use research (50.0).[73]
- On biosecurity, Canada lost points because personnel working with dangerous pathogens are only required to have background checks, and are not also required to have drug testing and psychological/mental fitness checks.[74]
- On dual-use research, Canada lost points because there is no legislation requiring DNA screening.[75]
- Canada’s biosecurity score improved by 3 points from 2019 to 2021. On other indicators Canada’s scores were static.[76]
- Canada has a perfect score on biosafety (100.0), but dropped points on biosecurity (86.7) and dual-use research (50.0).[73]
- In conversation, a biosecurity expert told me that he thought the Canadian system was the best in the world.
- Pannu et al. (2022), in a Science paper, highlight the Canadian system as “address[ing] key elements” of the problem of dual-use research.[77]
The system seems relatively comprehensive and flexible, and applies to emerging risks from bioengineered agents as well as existing risks.
- HPTAR apply to the private and the public sector and are legally binding.
- All work with pathogens requires a licence, and work with the most dangerous pathogens also requires a security clearance.
- Unlike many other countries where reporting of lab incidents is voluntary or retrospective, Canada has mandatory proactive reporting.[79]
- Pathogens need to have a risk assessment before you’re allowed to work with them, so even though the system is list-based it’s not so consequential if a list is missing something.[80]
- Any gain-of-function research must be reported in advance to the licence holder and Biological Safety Officer.[81]
There are weak indications that risk has in fact reduced
It’s hard to tell what the impact on risk has been, because there isn’t data from before the HPTA,[82] but there are some indications:
- The number of exposures has remained roughly constant even as the number of licences has grown significantly (with a peak in 2018 corresponding with a rise in licence numbers).[83]
- As of 2022, no incidents had been reported involving SSBAs since 2017-18.[84]
Other reasons
- The 2019 audit of the Biosecurity Program have been positive, though this was done by PHAC and so is quasi-internal[85]
- I’ve failed to find much criticism of the regime. The criticisms I did find were all from government sources, and tended to be procedural, rather than relating to fundamental inadequacies.
Strongest arguments against effectiveness
There are weak indications that incentives are more aligned with resolving compliance issues than with avoiding them in the first place.
- The main indicator the Centre uses to track “Public health risks associated with the use of pathogens and toxins are reduced” is % compliance issues in Canadian laboratories successfully responded to within established timelines.[86]
- On the one hand, this avoids incentivising the Centre not to find compliance issues, or incentivising them to find compliance issues which aren’t there.
- On the other, it also doesn’t create an incentive to actively reduce the rate of non-compliance.
- Notification is non-punitive.[87]
- On the one hand, this presumably leads to higher reporting rates.
- On the other hand, it doesn’t disincentivise incidents occurring in the first place.
- For extreme events, you want to avoid them happening in the first place, as you don’t get the chance to correct afterwards.
The median time between incident date and reporting date is 6 days.
- For serious incidents, this doesn’t seem fast enough to me.
A rate of ~5% of licence holders having an exposure incident every year seems high to me.
- It’s a bit difficult to know what the threshold should be though, and the rate of infections from those exposures is closer to 0.5%.[88]
At the time of the 2019 audit, there was the potential for COI in case of an incident at the National Microbiology Lab, which was also the only RG4 lab in Canada.
- In 2019, the status quo was that if there were an incident at the NML (which is part of PHAC, like the Centre), then the analyst who investigated it could end up being from the NML too.[89]
- I’m inferring that the NML was the only RG4 lab at that time (I think the direct information is classified): as of 2019 all RG4 pathogens in Canada were in one federal lab,[90] Ebola is an RG4 pathogen,[91] and the NML is a federal lab which conducted Ebola research projects before 2019.[92]
AI synthesised agents might be hard to manage safely.
- The person I spoke with at the Centre for Biosecurity thinks that HPTAR is fit for purpose when it comes to AI synthesis of biological agents, because pathogens need to have a risk assessment before you’re allowed to work with them[93]
- The person does note that completely unknown pathogens would be hard to deal with. The Centre tends to err on the side of caution, but it would be hard to justify risk group 4 without evidence
- I also guess that a large increase in pathogen risk assessment workload would not be resolved quickly (they’d need to hire new staff or learn to use AI to do it for them, and I don’t expect either of those would be speedy)
Other reasons
- At the time of the 2019 audit:
- The Centre’s risk register hadn’t been updated since 2016, though it was currently under review.[94]
- The rate of inspection for RG2 labs equated to an inspection once in over 20 years.[95]
- RG2 labs don’t deal with very dangerous pathogens, so from an underlying risk perspective this doesn’t seem that concerning to me.
- There were delays in the review of pathogen risk assessments, though the auditors thought it was very unlikely that this resulted in inappropriate containment levels in the interim “due to the Program’s close monitoring of emerging pathogens.”[96]
- The 2022 PHAC evaluation of the Human Pathogen and Toxins Act and Regulations Framework raised the following issues:[97]
- The WHO 2018 Joint External Evaluation noted “the need for an oversight framework on dual use in life sciences research”.[101]
Background info
History of the regime
- Motivation for the HPTA in 2009, according to the person I spoke with at the Centre for Biosecurity:[102]
- There was a recognition that there was a gap
- The previous legislation, HPIR, was just import
- The only kind of legislation which covered non-import work with pathogens was some occupational health and safety legislation
- The 2001 anthrax attacks prompted a recognition that using a criminal law statute (like HPTA) was perhaps the only way to appropriately deter and punish that type of behaviour
- There was a recognition that there was a gap
- Until the HPTR in 2015, the HPTA was not fully in force
- In developing the HPTR, PHAC considered continuing with no regulations, regulating under every part of HPTA, or minimal risk-based regulations. The latter approach was chosen[103]
Composition of the Centre for Biosecurity
- Office of Biosafety Programs and Planning (OBPP) - develops biosafety policies and risk assessment; focal point for international discussions[104]
- Office of Biosafety and Biocontainment Operations (OBBO) - administers HPTA/HPTR[105]
- Office of Pathogen Security (OPS) - strategic oversight and assistance on biosecurity[106]
- Office of Stakeholder Engagement and Regulatory Affairs (OSERA) - stakeholder comms[107]
Costs of the regime
- From 2016-17 to 2019-20, the Centre’s expenditure ranged from $8.1m to $9.7m. In 2020-21 this increased to $41.8m in the wake of Covid.[108]
- Canada has a One-for-One rule, which stipulates that:[109]
- When a new regulation is made an old one must be removed
- When a new regulation increases administrative burden, this should be offset to an equal amount
- In the case of HPTAR, the HPIR was repealed, and the rest of the administrative offset came from “the Health Portfolio's bank of administrative burden savings.”[110]
- In 2014, PHAC did a cost-benefit analysis on the HPTAR (which came into full force in 2015), and estimated that:
- In the first year, the incremental costs for regulated parties would total $2.4m, and government costs would be $6.8m.[111]
- Over the first 20 years, the incremental costs for regulated parties would be $19.4m, and $77.3m for the Canadian government.[112]
- Given the Centre’s actual expenditure from 2016 onwards, it seems likely that the government figure will be substantially exceeded.
- The cost-benefit analysis did not quantitatively estimate the benefits, but indicated that HPTAR would more than break even provided that they led to at least a 0.5% annual reduction in the risk of a SARS-like outbreak.[113]
Brief notes on some of Holden’s other questions
If a standard aims to reduce risks, to what extent did the standard get out ahead of/prevent risks, as opposed to being developed after relevant problems had already happened?
- Accidental and deliberate releases and pandemics had all happened before HPTAR
How involved are/were activists/advocates/people who are explicitly focused on public benefit rather than profits in setting standards? How involved are companies? How involved are people with reputations for neutrality?
- The initiative for HPTA seems to have come from government
- I haven’t come across anything which suggests otherwise
- In 2009 there was criticism that stakeholders were not properly consulted about the HPTA.[114]
Was there any influence of early voluntary standards on later government regulation?
- Not as far as I’m aware
Abbreviations
- BSO: Biological Safety Officer
- The Centre: the Centre for Biosecurity
- HPIR: Human Pathogens Importation Regulations
- HPTA: Human Pathogens and Toxins Act
- HPTAR: Human Pathogens and Toxins Act and Regulations
- HPTR: Human Pathogens and Toxins Regulations
- LAI: Laboratory-Associated Infection
- SSBA: Security Sensitive Biological Agents
Select bibliography
Regulatory documents
Useful Centre for Biosecurity webpages
- Centre for Biosecurity Compliance and Enforcement Policy
- Licensing program
- HPTA
- Security Sensitive Biological Agents (SSBAs)
Evaluations of Canadian biorisk regulation
- Evaluation of the Biosecurity Program 2009–10 to 2013–14, Evaluation Directorate, 2014.
- International Health Regulations – Joint External Evaluation of Canada Self-Assessment Report, PHAC, 2018.
- Audit of the Biosecurity Program at the Public Health Agency of Canada, Office of Audit and Evaluation, 2019.
- 2021-GHS-Index-April-2022, downloaded from https://www.ghsindex.org/report-model/.
- 2021 GHS Index Country Profile for Canada
- Country score justifications and references: Canada, GHS Index, 2021.
- Evaluation of the Human Pathogens and Toxins Act and Regulations Framework 2015-16 to 2020-21, Office of Audit and Evaluation, 2022.
- Departmental Results Reports, PHAC.
Other useful sources
- Regulatory Impact Analysis Statement for the Human Pathogens and Toxins Act and Regulations (HPTAR), PHAC, 2014.
- Laboratory exposure to human pathogens and toxins, Canada 2020, CCDR, 2021
- Laboratory exposure to human pathogens and toxins, Canada 2021, CCDR, 2022
- ^
“The CBS is used by the PHAC and the Canadian Food Inspection Agency (CFIA) to verify the ongoing compliance of regulated facilities with a licence for controlled activities with human pathogens and toxins or importing or transferring terrestrial animal pathogens.” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/human-pathogens-toxins-act.html#s4
- ^
“The Canadian Biosafety Handbook (CBH) is a companion document to the CBS that provides guidance on how to achieve the biosafety and biosecurity requirements outlined in the CBS.” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/human-pathogens-toxins-act.html#s4
- ^
- ^
“Besides its regulatory and inspection mandate, the Centre has a law enforcement mandate.” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/compliance-enforcement/centre-biosecurity-compliance-enforcement-policy.html
- ^
- ^
“There are approximately 1,000 licence holders in Canada that have one or multiple laboratories under their management.” Audit of the Biosecurity Program at the Public Health Agency of Canada, Office of Audit and Evaluation, 2019
- ^
“the Minister of Health may use an Interim Order under section 67 of the HPTA to prescribe a new and/or emerging risk group 3 or 4 human pathogen or toxin as an SSBA.” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/biosecurity/biosecurity-frequently-asked-questions.html
- ^
- ^
“the Minister of National Defence may refuse to disclose any information the disclosure of which could reasonably be expected to be injurious to the defence or security of Canada or of a state allied or associated with Canada.” https://laws-lois.justice.gc.ca/eng/acts/H-5.67/FullText.html#h-255104, Section 38. “the Minister of National Defence may refuse to disclose any information the disclosure of which could reasonably be expected to be injurious to the defence or security of Canada or of a state allied or associated with Canada.” https://laws-lois.justice.gc.ca/eng/acts/H-5.67/FullText.html#h-255104, Section 40.
- ^
“The health and economic damages related to a SARS-type outbreak are more than 200 times the annual costs of these proposed Regulations. Even a 0.5% reduction in the annual risk of such an event would, therefore, more than outweigh the costs of these proposed Regulations.” Regulatory Impact Analysis Statement for the Human Pathogens and Toxins Act and Regulations (HPTAR), PHAC, 2014.
- ^
Evaluation of the Biosecurity Program 2009–10 to 2013–14, Evaluation Directorate, 2014.
- ^
“"The Human Pathogens and Toxins Act and the Human Pathogens and Toxins Regulations do not require dangerous pathogens and toxins to be consolidated in a minimum number of facilities in Canada. Nonetheless, some dangerous pathogens have been consolidated to a minimum number of facilities, for example all RG4 pathogens are consolidated in one jointly operated federal facility in Canada and efforts are underway to reduce the number of facilities in Canada that contain polio virus.” International Health Regulations – Joint External Evaluation of Canada Self-Assessment Report, PHAC, 2018.
- ^
“In Budget 2021, significant investments of over $3 billion were made to advance the biomanufacturing and life sciences sector in Canada. This has led to new policy and legislative challenges, such as the need to consider licensing private sector containment level 4 (CL4) laboratories, including in academic institutions, which has never previously been done in Canada.” Evaluation of the Human Pathogens and Toxins Act and Regulations Framework 2015-16 to 2020-21, Office of Audit and Evaluation, 2022.
- ^
From a conversation I had with a person who works at the Centre for Biosecurity.
- ^
- ^
“[P]roduce, in respect of a human pathogen or toxin, means to create it by any method or process, including
(a) by manufacturing, cultivating, developing, reproducing or synthesizing it; or
(b) by converting or refining a substance, micro-organism, nucleic acid or protein, or by using any other means of altering its physical or chemical properties.”
https://laws-lois.justice.gc.ca/eng/acts/H-5.67/FullText.html
- ^
- ^
https://laws-lois.justice.gc.ca/eng/acts/H-5.67/FullText.html#h-255104, Schedules 2-4. Group 2 is “moderate risk to the health of individuals and a low risk to public health”; group 3 is “high risk to the health of individuals and a low risk to public health”; group 4 is “high risk to the health of individuals and a high risk to public health”.
- ^
- ^
“Security sensitive biological agents, or SSBAs, are a subset of Risk Group 3 and 4 human pathogens and prescribed toxins that are included in Schedule 1 of the Human Pathogens and Toxins Regulations (HPTR) and also on the Australia Group "List of Human and Animal Pathogens and Toxins for Export Control" (AG List).” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/biosecurity/biosecurity-frequently-asked-questions.html
- ^
https://laws-lois.justice.gc.ca/eng/acts/H-5.67/FullText.html, Sections 9-11.
- ^
“the Minister of Health may use an Interim Order under section 67 of the HPTA to prescribe a new and/or emerging risk group 3 or 4 human pathogen or toxin as an SSBA.” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/biosecurity/biosecurity-frequently-asked-questions.html
- ^
“ePATHogen is the Public Health Agency of Canada’s biological agent search tool. ePATHogen contains a searchable list of biological agents with their associated human and animal risk group classifications, as well as the associated containment level (CL), Security Sensitive Biological Agent (SSBA) status, regulatory authority, and containment considerations. A list of regulated toxins is also included.” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/human-pathogens-toxins-act.html#s4
- ^
“If a biological agent of interest is not listed in ePATHogen, a pathogen risk assessment will need to be conducted to determine the human and animal risk group classifications. Please complete the pathogen risk assessment template and email it to pathogens.pathogenes@phac-aspc.gc.ca for validation.” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/human-pathogens-toxins-act.html#s4. There’s further guidance on this risk assessment here.
- ^
- ^
From a conversation I had with a person who works at the Centre for Biosecurity.
- ^
“the Minister of National Defence may refuse to disclose any information the disclosure of which could reasonably be expected to be injurious to the defence or security of Canada or of a state allied or associated with Canada.” https://laws-lois.justice.gc.ca/eng/acts/H-5.67/FullText.html#h-255104, Section 38. “the Minister of National Defence may refuse to disclose any information the disclosure of which could reasonably be expected to be injurious to the defence or security of Canada or of a state allied or associated with Canada.” https://laws-lois.justice.gc.ca/eng/acts/H-5.67/FullText.html#h-255104, Section 40.
- ^
- ^
“It should be noted that there is no fee for licensing activities under the HPTA (including applying for, amending, renewing, etc.).” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/licensing-program.html
- ^
“Separate licences will grant authority to perform activities with:
all RG2 human pathogens, prions, and all toxins that are not SSBAs in a broad area of the organisation,
specific prescribed toxin(s) in a defined part of a facility,
specific list of RG3 pathogens in a defined laboratory space (the list may or may not include SSBA), and
specific list of RG4 pathogens in a defined laboratory space (the list may or may not include SSBA).” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/licensing-program.html
- ^
“The maximum term of a Pathogen and Toxin Licence varies depending on the risk group of the pathogen(s). The maximum licence term is 5 years for risk group 2 pathogens, prions, and non-SSBA toxins. The maximum term is 3 years for risk group 3 pathogens and SSBA toxins and a 1 year term for risk group 4 pathogens.” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/licensing-program.html
- ^
“Licences are subject to various conditions. The conditions include an obligation for ongoing compliance with the applicable requirements described in the Canadian Biosafety Standard (CBS).” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/licensing-program.html. For all conditions, see Section 4 of https://laws-lois.justice.gc.ca/eng/regulations/SOR-2015-44/FullText.html
- ^
From a conversation I had with a person who works at the Centre for Biosecurity.
- ^
“A licence holder shall establish and maintain a list of all persons authorized by the licence holder to access the facility to which the licence applies, including persons holding a security clearance for that facility and visitors. The licence holder shall provide the Minister with that list if requested to do so.” https://laws-lois.justice.gc.ca/eng/acts/H-5.67/FullText.html#h-255104 , Section 31
- ^
https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/licensing-program.html. Scientific research is defined here.
- ^
- ^
- ^
“There is a requirement to obtain a HPTA Security Clearance if you work with, or have access to SSBAs.” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/licensing-program.html
- ^
“Security sensitive biological agents, or SSBAs, are a subset of Risk Group 3 and 4 human pathogens and prescribed toxins that are included in Schedule 1 of the Human Pathogens and Toxins Regulations (HPTR) and also on the Australia Group "List of Human and Animal Pathogens and Toxins for Export Control" (AG List).” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/biosecurity/biosecurity-frequently-asked-questions.html
- ^
“The Agency has prepared a list of toxins that will require an HPTA Security Clearance if an individual is in possession of a toxin in an amount that exceeds the threshold limits stated in section 10 of the HPTR. Individuals working with toxins in quantities less than the indicated trigger quantity will not require an HPTA Security Clearance.” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/biosecurity/biosecurity-frequently-asked-questions.html
- ^
“Individuals who do not hold an HPTA Security Clearance may access part(s) of a facility where controlled activities with SSBAs are authorized if they are accompanied and supervised by an individual who holds an HPTA Security Clearance. The HPTR specify that accompaniment and supervision of non-cleared individuals means a one to one ratio with activities monitored at all times by an individual who holds an HPTA Security Clearance.” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/biosecurity/biosecurity-frequently-asked-questions.html
- ^
“Individuals who have had their HPTA Security Clearance suspended or revoked cannot access the part(s) of a facility where controlled activities with SSBAs are authorized, even when accompanied and supervised.” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/biosecurity/biosecurity-frequently-asked-questions.html
- ^
“There will be no fee charged by the Agency to apply for an HPTA Security Clearance however there will be a fee associated to the taking of fingerprints by RCMP accredited agencies… The cost for this service will vary from one agency to another (approximately $30-$150).” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/biosecurity/biosecurity-frequently-asked-questions.html
- ^
- ^
For all functions, see https://laws-lois.justice.gc.ca/eng/regulations/SOR-2015-44/FullText.html, Section 9
- ^
- ^
“A licence holder must — if their licence authorizes controlled activities in respect of a human pathogen that falls into Risk Group 3 or Risk Group 4 or in respect of a prescribed toxin — notify the Minister before they make any change to the physical structure of the facility, to any equipment or to the standard operating procedures that could affect biocontainment.” https://laws-lois.justice.gc.ca/eng/regulations/SOR-2015-44/FullText.html Section 6
- ^
“Launched in 2015, the LINC system is unique in that it is one of the first comprehensive national surveillance systems to provide a systematic framework for reporting human pathogen and toxin exposures and LAIs in various settings. In contrast, national reporting requirements for LAIs in other countries is often voluntary or conducted via retrospective survey.” Evaluation of the Human Pathogens and Toxins Act and Regulations Framework 2015-16 to 2020-21, Office of Audit and Evaluation, 2022.
- ^
- ^
For example, an incident report involving polio would be followed up within 24 hours. Other things requiring rapid turnaround include inadvertent release, confirmed laboratory-associated infections, risk group 4 agents, and anything which could generate media attention. From a conversation I had with a person who works at the Centre for Biosecurity.
- ^
From a conversation I had with a person who works at the Centre for Biosecurity. There are some no contravention clauses in HPTA. Provided that you transfer or dispose of it in accordance with the Act, you don’t contravene the Act for illegal possession of a pathogen or toxin because of inadvertent production or an update to the lists. https://laws-lois.justice.gc.ca/eng/acts/H-5.67/FullText.html, Schedules 11 and 12.
- ^
“A person who is conducting controlled activities authorized by a licence must notify the licence holder and the biological safety officer, in writing, whenever they intend to do any of the following:
(a) increase the virulence or pathogenicity of a human pathogen;
(b) increase the communicability of a human pathogen;
(c) increase the resistance of a human pathogen to preventive or therapeutic treatments; or
(d) increase the toxicity of a toxin.” https://laws-lois.justice.gc.ca/eng/regulations/SOR-2015-44/FullText.html Schedule 5
“Mandatory reporting to the license holder and the BSO of any activities that could result in the creation of a human pathogen with increased virulence, pathogenicity, or communicability, that is resistant to preventative or therapeutic treatments, or produces a toxin with increased toxicity (HPTR 5). This requirement also applies to work that is not considered dual-use.“ https://www.canada.ca/en/public-health/programs/consultation-biosafety-guideline-dual-use-life-science-research/document.html
- ^
- ^
- ^
https://laws-lois.justice.gc.ca/eng/acts/H-5.67/FullText.html#h-255104, Section 59. You’re also not liable for inadvertently producing a regulated substance if you take appropriate steps. Section 12(4).
- ^
- ^
“(a) examine the place — including any building — or conveyance and any material or equipment found there;
(b) require any person in the place or conveyance to produce, in the manner and form requested by the inspector, any material or equipment found there;
(c) seize and detain for any time that may be necessary any material, equipment or document found there, or any conveyance;
(d) open and examine any receptacle or package found there;
(e) take, or require any person in the place or conveyance to produce, free of charge, a sample of any material found there;
(f) direct the owner or the person having possession, care or control of any material, equipment or document found in the place or conveyance — or of the conveyance — to move it or, for any time that may be necessary, not to move it or to restrict its movement;
(g) conduct, or require any person in the place or conveyance to conduct, any test or analysis or take any measurement of any material or equipment found there;
(h) take photographs or make recordings or sketches;
(i) examine and make copies, in whole or in part, of any book, document or other record found there;
(j) require any person in the place or conveyance to produce any book, document or other record found there for examination or copying;
(k) use or cause to be used any computer system or other device found there to examine information that is contained in or available to the computer system or device;
(l) reproduce any information in the form of a printout or other intelligible output for examination or copying; and
(m) use or cause to be used any copying equipment.”
https://laws-lois.justice.gc.ca/eng/acts/H-5.67/FullText.html#h-255104, Section 40
- ^
“Licensed facilities handling SSBAs and RG3 and RG4 human pathogens are inspected at least once during the term of their licence, every one to three years.” Evaluation of the Human Pathogens and Toxins Act and Regulations Framework 2015-16 to 2020-21, Office of Audit and Evaluation, 2022.
- ^
From a conversation I had with a person who works at the Centre for Biosecurity.
- ^
“ While facilities working with RG2 pathogens are traditionally considered lower-risk, evidence gathered to date through mandatory incident reporting requirements suggests that they account for a disproportionate number of exposure notifications.” Evaluation of the Human Pathogens and Toxins Act and Regulations Framework 2015-16 to 2020-21, Office of Audit and Evaluation, 2022.
- ^
From a conversation I had with a person who works at the Centre for Biosecurity.
- ^
“the Minister of National Defence may refuse to disclose any information the disclosure of which could reasonably be expected to be injurious to the defence or security of Canada or of a state allied or associated with Canada.” https://laws-lois.justice.gc.ca/eng/acts/H-5.67/FullText.html#h-255104, Section 40.
- ^
2018-19: 88%, 2019-20: 98%, 2020-21: 100%, 2021-22: 98%. Departmental Results Reports, PHAC.
- ^
From a conversation I had with a person who works at the Centre for Biosecurity.
- ^
“Since the current Human Pathogens and Toxins Act came into force in 2009, there has been only one prosecution.” Evaluation of the Biosecurity Program 2009–10 to 2013–14, Evaluation Directorate, 2014. The person I spoke with at the Centre for Biosecurity told me in 2023 that there had still only been one prosecution. The single case is that of scientist Klaus Nielsen, who tried to board a planen to China with live brucella bacteria, and was sentenced to two years in prison. https://ottawacitizen.com/news/local-news/canadian-government-scientist-who-smuggled-bacteria-in-carry-on-luggage-gets-prison-time
- ^
From a conversation I had with a person who works at the Centre for Biosecurity.
- ^
Evaluation of the Biosecurity Program 2009–10 to 2013–14, Evaluation Directorate, 2014.
- ^
“There is a lack of European-wide harmonized practical guidance on how to implement the European Directives on biological agents and GMMs. A few EU Member States have developed their own national guidance based on the EC Directives. In other cases, these gaps are filled by e.g. US Biosafety in Microbiological and Biomedical Laboratories (BMBL) and Canadian guidelines”. Johnson and Casagrande, ‘Comparison of International Guidance for Biosafety Regarding Work Conducted at Biosafety Level 3 (BSL-3) and Gain-ofFunction (GOF) Experiments’, Applied Biosafety, 2016.
- ^
E.g. “In 2008, Russia received assistance from the Canadian Association for Biological Safety to train instructors for biosafety programs. WHO and US biosafety documents were translated and used for training purposes”. “Through this partnership [the Global Partnership Against the Spread of Weapons and Materials of Mass Destruction], Canada provided Russia assistance in improving biosafety and biosecurity standards. Canada has translated biosafety training programs and documents into Russian for more widespread use”. National biosafety systems: Case studies to analyze current biosafety approaches and regulations for Brazil, China, India, Israel, Pakistan, Kenya, Russia, Singapore, the United Kingdom, and the United States, UPMC Center for Health Security, 2016.
- ^
- ^
2021-GHS-Index-April-2022, downloaded from https://www.ghsindex.org/report-model/.
- ^
- ^
Country score justifications and references: Canada, GHS Index, 2021.
- ^
Country score justifications and references: Canada, GHS Index, 2021.
- ^
- ^
Pannu et al. 2022: paragraph 4. Thanks to Bill Anderson-Samways for this reference. See this section of his case study on FSAP.
- ^
Thanks to Bill Anderson-Samways for making this salient to me. See this section of his case study on FSAP.
- ^
“Launched in 2015, the LINC system is unique in that it is one of the first comprehensive national surveillance systems to provide a systematic framework for reporting human pathogen and toxin exposures and LAIs in various settings. In contrast, national reporting requirements for LAIs in other countries is often voluntary or conducted via retrospective survey.” Evaluation of the Human Pathogens and Toxins Act and Regulations Framework 2015-16 to 2020-21, Office of Audit and Evaluation, 2022.
- ^
“If a biological agent of interest is not listed in ePATHogen, a pathogen risk assessment will need to be conducted to determine the human and animal risk group classifications. Please complete the pathogen risk assessment template and email it to pathogens.pathogenes@phac-aspc.gc.ca for validation.” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/human-pathogens-toxins-act.html#s4. There’s further guidance on this risk assessment here.
- ^
“A person who is conducting controlled activities authorized by a licence must notify the licence holder and the biological safety officer, in writing, whenever they intend to do any of the following:
(a) increase the virulence or pathogenicity of a human pathogen;
(b) increase the communicability of a human pathogen;
(c) increase the resistance of a human pathogen to preventive or therapeutic treatments; or
(d) increase the toxicity of a toxin.” https://laws-lois.justice.gc.ca/eng/regulations/SOR-2015-44/FullText.html Schedule 5
“Mandatory reporting to the license holder and the BSO of any activities that could result in the creation of a human pathogen with increased virulence, pathogenicity, or communicability, that is resistant to preventative or therapeutic treatments, or produces a toxin with increased toxicity (HPTR 5). This requirement also applies to work that is not considered dual-use.“ https://www.canada.ca/en/public-health/programs/consultation-biosafety-guideline-dual-use-life-science-research/document.html
- ^
From a conversation I had with a person who works at the Centre for Biosecurity.
- ^
Evaluation of the Human Pathogens and Toxins Act and Regulations Framework 2015-16 to 2020-21, Office of Audit and Evaluation, 2022.
- ^
“Data outlining the number of missing, lost, or stolen pathogen or toxin incidents involving Security Sensitive Biological Agents (SSBAs), which have the highest potential of being used for malicious intent, shows that there have been no incidents reported since 2017-18.” Evaluation of the Human Pathogens and Toxins Act and Regulations Framework 2015-16 to 2020-21, Office of Audit and Evaluation, 2022.
- ^
The 2019 audit of the Biosecurity Program “found that effective management controls were in place to ensure a safe and secure regime to protect the health and safety of the public against risks posed by the use of human pathogens and toxins.” Audit of the Biosecurity Program at the Public Health Agency of Canada, Office of Audit and Evaluation, 2019.
- ^
2018-19: 88%, 2019-20: 98%, 2020-21: 100%, 2021-22: 98%. Departmental Results Reports, PHAC.
- ^
From a conversation I had with a person who works at the Centre for Biosecurity.
- ^
Evaluation of the Human Pathogens and Toxins Act and Regulations Framework 2015-16 to 2020-21, Office of Audit and Evaluation, 2022.
- ^
“We noted that the current pool of designated analysts was housed within the NML and CFIA’s National Centre for Foreign Animal Disease (NCFAD). In the event of a situation at the NML, or situation involving one of its employees, an analysis of pathogens by a designated analyst would be required; however, using an NML-designated analyst could give rise to the appearance of a conflict of interest, which could undermine penal enforcement efforts. Although this risk was mitigated by the Program’s ability to use the NCFAD laboratory for appropriate sample storage, analysis, and detention, as well as close monitoring of the NML through yearly inspections and ongoing support, the lack of a contingency plan could result in the Program being reactive, leading to confusion and inefficiency, and affecting the Centre’s reputation as an effective regulator.” Audit of the Biosecurity Program at the Public Health Agency of Canada, Office of Audit and Evaluation, 2019
- ^
“"The Human Pathogens and Toxins Act and the Human Pathogens and Toxins Regulations do not require dangerous pathogens and toxins to be consolidated in a minimum number of facilities in Canada. Nonetheless, some dangerous pathogens have been consolidated to a minimum number of facilities, for example all RG4 pathogens are consolidated in one jointly operated federal facility in Canada and efforts are underway to reduce the number of facilities in Canada that contain polio virus.” International Health Regulations – Joint External Evaluation of Canada Self-Assessment Report, PHAC, 2018.
- ^
- ^
- ^
From a conversation I had with a person who works at the Centre for Biosecurity.
- ^
“We noted that the Centre’s risk register had not been updated since February 2016. However, at the time of the audit, the Centre was developing its strategic plan and updating its risk register.” Audit of the Biosecurity Program at the Public Health Agency of Canada, Office of Audit and Evaluation, 2019
- ^
“In 2017-18, the program inspected 4% of the RG2 population of 885 licenses, for a total of 45 inspections. At this rate, it would take over 20 years to inspect all RG2 license holders at least once.” Audit of the Biosecurity Program at the Public Health Agency of Canada, Office of Audit and Evaluation, 2019
- ^
“Delays in assessing the risk group of pathogens may have resulted in regulated parties performing controlled activities within inappropriate containment levels; however, this is highly unlikely due to the Program’s close monitoring of emerging pathogens.” Audit of the Biosecurity Program at the Public Health Agency of Canada, Office of Audit and Evaluation, 2019
- ^
“Issues were raised regarding security clearance requirements, including expanding the criteria for who should have a security clearance, beyond those who work with or have access to RG3 and RG4 pathogens, as well as increasing the level of scrutiny prior to issuing a security clearance to a foreign national. Licensing challenges were also raised, including clearly defining roles and responsibilities between Biological Safety Officers and Licence Holders. Addressing administrative burden for regulated organizations in the areas of licensing, security clearances, and inspections was also identified as a key area of focus (e.g., streamlining application process and the information needed from applicants and harmonizing HPTA requirements with the Health of Animals Act (HAA) requirements. Finally, the appropriateness of using virtual inspections and how they could complement existing physical inspections was identified as an area for further consideration.” Evaluation of the Human Pathogens and Toxins Act and Regulations Framework 2015-16 to 2020-21, Office of Audit and Evaluation, 2022.
- ^
“For the most part, internal and external key informants agreed that security clearance requirements should be expanded to include more individuals working with or having access to human pathogens and toxins… Among others, this would apply to IT personnel, inspectors, and regulatory officials with access to floor plans or HPT inventory records.” Evaluation of the Human Pathogens and Toxins Act and Regulations Framework 2015-16 to 2020-21, Office of Audit and Evaluation, 2022.
- ^
“Most federal stakeholders agreed that there is a need for continuous vetting of existing security clearances, typically issued for a five-year period, citing that a person’s situation or motivations can change over time (e.g., financial or marital situation, mental health-related issues, such as anger and resentment, sharing knowledge of their work when being approached or harassed by an external threat). They felt that a flexible system could be considered, where risk indicators can trigger renewed vetting or that there should be open communication between the clients and PHAC, with incidents being reported and reviewed accordingly.” Evaluation of the Human Pathogens and Toxins Act and Regulations Framework 2015-16 to 2020-21, Office of Audit and Evaluation, 2022.
- ^
“In Budget 2021, significant investments of over $3 billion were made to advance the biomanufacturing and life sciences sector in Canada. This has led to new policy and legislative challenges, such as the need to consider licensing private sector containment level 4 (CL4) laboratories, including in academic institutions, which has never previously been done in Canada.” Evaluation of the Human Pathogens and Toxins Act and Regulations Framework 2015-16 to 2020-21, Office of Audit and Evaluation, 2022.
- ^
- ^
From a conversation I had with a person who works at the Centre for Biosecurity.
- ^
Regulatory and non-regulatory options considered, Regulatory Impact Analysis Statement for the Human Pathogens and Toxins Act and Regulations (HPTAR), PHAC, 2014.
- ^
“Office of Biosafety Programs and Planning (OBPP) - develops biosafety program and policy components to protect against existing and emerging risks; establishes and maintains biosafety risk assessments and biocontainment directives; acts as the focal point for the Agency for key international discussions, such as the Biological and Toxin Weapons Convention (BTWC) and the Australia Group, to ensure that international discussions are considered for domestic policy, and vice-versa.” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/about-centre-biosecurity.html
- ^
“Office of Biosafety and Biocontainment Operations (OBBO) - administers and enforces the HPTA, HPTR, and select sections of the HAA/HAR; issues HPTA Licences, evidence of compliance with containment standards, and certifications for those labs using higher risk human pathogens or toxins, as required by the HPTR; verifies compliance (both microbiological and engineering) using inspections, audits, etc.; advances biocontainment engineering science to identify knowledge gaps and to inform biosafety standards and guidelines.” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/about-centre-biosecurity.html
- ^
“Office of Pathogen Security (OPS) - develops and manages strategic partnerships to facilitate a whole of Government approach to pathogen oversight in Canada and alignment internationally where possible; provides technical assistance and resources to domestic and international partners and stakeholders in the area of laboratory biosecurity with a view to mitigating deliberate misuse of pathogens and toxins; facilitates and implements innovative information technology solutions to support administration and compliance activities for the Centre at the border and domestically.” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/about-centre-biosecurity.html
- ^
“Office of Stakeholder Engagement and Regulatory Affairs (OSERA) - responsible for the development and comprehensive lifecycle management of the Acts and Regulations administered by the Centre; serves as the coordination and briefing unit for all regulatory initiatives involving or implicating the Agency; develops strong and sustainable relationships with stakeholders through outreach, targeted stakeholder communications, engagement and consultation activities; provides secretariat support to external advisory committees.” https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/about-centre-biosecurity.html
- ^
Evaluation of the Human Pathogens and Toxins Act and Regulations Framework 2015-16 to 2020-21, Office of Audit and Evaluation, 2022.
- ^
“The One-for-One rule aims to reduce regulatory burden in two ways: remove a regulation when a new one is set in place; and when a new or amended regulation increases administrative burden on business, there should be an offset with an equal amount of administrative burden cost on business.” Evaluation of the Biosecurity Program 2009–10 to 2013–14, Evaluation Directorate, 2014.
- ^
Evaluation of the Biosecurity Program 2009–10 to 2013–14, Evaluation Directorate, 2014.
- ^
“It is estimated that the incremental cost of compliance with these Regulations would be $2.41 million for all regulated parties, representing approximately 8 500 laboratories, in the first year following the coming into force of the HPTR. The Government would incur program administration costs of $6.82 million in this first year.” Regulatory Impact Analysis Statement for the Human Pathogens and Toxins Act and Regulations (HPTAR), PHAC, 2014.
- ^
Regulatory Impact Analysis Statement for the Human Pathogens and Toxins Act and Regulations (HPTAR), PHAC, 2014.
- ^
“The health and economic damages related to a SARS-type outbreak are more than 200 times the annual costs of these proposed Regulations. Even a 0.5% reduction in the annual risk of such an event would, therefore, more than outweigh the costs of these proposed Regulations.” Regulatory Impact Analysis Statement for the Human Pathogens and Toxins Act and Regulations (HPTAR), PHAC, 2014.
- ^
“In 2009, when the Human Pathogens and Toxins Act (Bill C-11) was first tabled in Parliament, the Public Health Agency was criticised for not adequately involving stakeholders during the development of the Act.” Evaluation of the Biosecurity Program 2009–10 to 2013–14, Evaluation Directorate, 2014.

Executive summary: Canadian regulations for biorisk seem comprehensive and effective at compliance, though incentives may focus more on resolving issues than prevention, and gaps likely remain for extreme risks.
Key points:
This comment was auto-generated by the EA Forum Team. Feel free to point out issues with this summary by replying to the comment, and contact us if you have feedback.