Key points
- This is a condensed version of a university assignment (health technology proposal, Australia-based) I wrote recently. I'm not an expert on this (the basis for writing this is as a medical doctor studying a public health degree). I'm uncertain whether this is worthwhile, I am mostly looking for people with more experience /research capacity to tell me whether or not this might be useful.
- My inclination is that further research into this area could be really useful as an option for some of increasingly untreatable "superbugs", or as a short-term treatment option for novel epi-/pandemics while other responses are mounted.
- Extracorporeal photopheresis, or irradiation of small amounts of blood outside of the body, is a potential treatment modality for infection. Similar methods are used to treat immune system overactivation. Perhaps surprisingly, the over-activation of the immune system is very important to treat in severe infections. Sepsis and SARS are referring to the immune response to pathogens, not to the pathogen itself. In this report I focus on influenza, but this could be simply replaced with almost any virus or bacterial infection. (see sections on Claimed benefits of the technology, ECP safety, Dose and duration)
- ECP has limited, yet promising, evidence. It was mostly investigated before antibiotics dominated the field (then forgotten it seems). Existing data describes 151 cases of acute bacterial infection in the blood (bacteraemia), with a 100% non-progression rate to septicaemia in the treatment group (Miley, 1942). A further study demonstrated 56% of presently septic patients recovered uneventfully following UV therapy (Miley, 1944). (See ECP effectiveness)
- My areas of greatest uncertainty here are the clinical benefit (as the studies are from the 1940s and don't seem to have been picked up again since) and the costings (this is the first time I've attempted something like this and I just used a poor proxy of hospital days instead of a more robust measure like DALYs).
- The incremental cost effectiveness ratio is thus limited due to few studies and my limited understanding of costing complex things like disease. As it stands, for influenza the ICER I have calculated is 5.67 for the first year in Australia. Obviously this would vary enormously based on other settings and pathogens. (See Economic evaluation)
- Everything below here is included for further information, I've tried to summarise my queries to readers in the Key Points section.
1. PICO
Population
The proposed patient population is:
Patients with laboratory confirmed influenza infection (of any strain) of any age AND any of those who:
- Have established complications
- need to be admitted to hospital for management of influenza
- Have moderate-severity or high-severity community-acquired pneumonia, during the influenza season
- Are at high risk of severe influenza, defined as
- adults aged 65 years or older
- pregnant women
- people with the following conditions:
- heart disease
- Down syndrome
- obesity (body mass index [BMI] 30 kg/m2 or more)
- chronic respiratory conditions
- severe neurological conditions
- immune compromise
- other chronic illnesses
- Aboriginal and Torres Strait Islander people of any age
- children aged 5 years or younger
- residents of aged-care facilities or long-term residential facilities
- homeless people (Therapeutic Guidelines, 2022)
This proposed patient population is based on the combination of recommendations made within guidelines on antiviral use for influenza treatment and evidence from the literature regarding morbidity and mortality.
Intervention
Immunophototherapy, or UV irradiation treatment of the person’s blood to increase immune response to influenza infection.
This involves a three step process: sourcing 5% of a patient’s blood volume (3.5mL/kg) via intravenous cannulation, and separation of cells via apheresis. A photosensitising agent methoxsalen is added and the cells are exposed to ultraviolet A light through the PIT machine. The treated cells are then returned to the patient. This is a form of autologous blood transfusion, where the donor and recipient are the same person.
This process has previously been approved for another indication by both the Medical Services Advisory Committee and the Pharmaceutical Benefits Advisory Committee.
Comparator
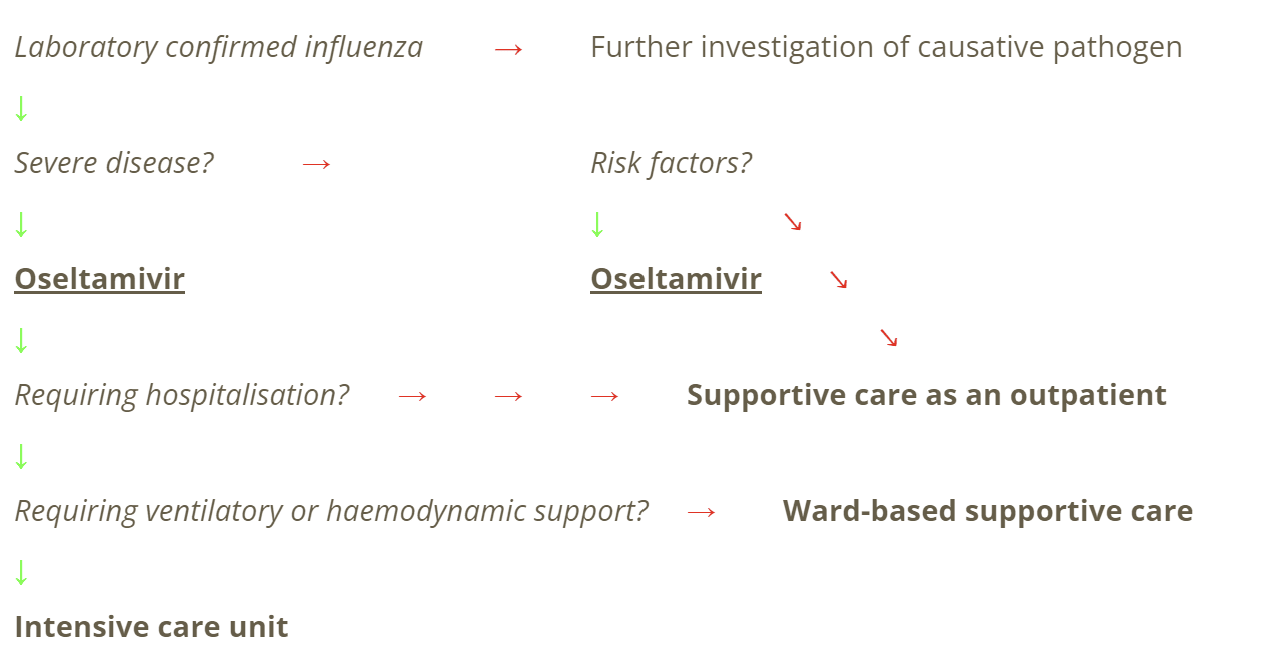
If influenza infection is contracted, people may be offered a course of Tamiflu (oseltamivir), a neuraminidase inhibitor. This is generally 75 mg twice daily, for 5 days as an oral tablet. It is most effective if taken within the first 48 hours (Robson C, Baskar SR, Booy R, Ferguson PE, Gilroy N, Kok J, et al., 2022).
Oseltamivir is mentioned in the guidelines as an option for people with established complications. It is “to be considered” in people at high risk of severe influenza. It is not recommended at all for healthy adults. It is not funded by the Pharmaceutical Benefits Scheme.
In serious cases requiring hospitalisation, supportive care is the mainstay of current treatment, with escalation to ventilator and/or intensive care with haemodynamic support in severe cases. Oseltamivir is often given, however its efficacy is decreased the later it is given. Increased doses or prolonged durations are not recommended (Therapeutic Guidelines, 2022).
While vaccination is the principal way to prevent severe infection, it must be given before infection occurs. Influenza vaccination coverage in Australia is low, meaning a majority of people are not protected in this way (Sullivan, 2020). Therefore, it is not a suitable comparator in this case.
Outcomes
Primary outcomes will be the number of severe cases of influenza requiring hospitalisations, duration of hospital treatment, mortality rate, side effects and long term effects including decreased Quality Adjusted Life Years.
Secondary outcome of interest is the effect on resistance via virological surveillance of mutating strains. This may be demonstrated by reducing the actual versus expected number of Influenza pandemic/ epidemics in Australia. This is of interest as epidemics are more severe if they are strains resistant to neuraminidase inhibitors, and resistance has developed in patients treated with neuraminidase inhibitors.
2. The proposed technology or service and its proposed place in clinical practice
Clinical issue
- Influenza (HxNx) is a common form of viral infection which predominantly affects the respiratory system. Influenza affects people of any age, but is particularly severe in the very young, those over 65 and those living with immunocompromised. It was the most common cause of infectious disease notification in Australia every year until 2019 (since COVID-19 has increased) (Sullivan, 2020). It is a notifiable disease which is highly variable in incidence from year to year; from 10 to 125 to hospitalisations per 1,000 people, and mortality rate; from 346 to 1776 people (1.63 to 7.22 per 100,000 people) since 2000 (AIHW, 2023).
- Some forms of influenza, including swine flu (H1N1) and bird flu (H7N9), may be recognisable due to their pandemic potential pathogen status. There are common types of Influenza, known as A, B, and C, representing different combinations of glycoproteins hemagglutinin (H) and neuraminidase (N). The seasonal nature of the flu virus derives from the tendency for antigenic shift and drift in these glycoproteins (McKimm-Breschkin, 2013). This frequent adaptation of the surface proteins of the virus means that it can evade the immune system in those who have had previous infection.
- Due to its ability to evolve, yearly vaccination is the main measure to prevent infection. This is provided free to many high-risk people through the National Immunisation program (Sullivan et al, 2020).
- Influenza infection is most commonly treated with supportive care, including rest and antipyretic therapy. Vulnerable subpopulations may be offered antiviral therapy with neuraminidase inhibitors (oseltamivir). Despite its inclusion on the TGA, the PBAC refused to list oseltamivir on the PBS (PBS, 2008). It remains on the eTG, with a disclaimer “despite limited evidence of benefit.” There is not currently an evidence-based post exposure influenza treatment which has government funding (Robson C et al., 2022). ETG recommends this for people:
- With established complications
- With moderate to severe community acquired pneumonia during flu season
- requiring hospitalisation
- Who are pregnant or otherwise at high risk of severe infection
- The proposed patient population that would benefit most from irradiation therapy are patients with high risk of severe outcomes from influenza, particularly those in areas where the potential for epidemic spread is high, such as cities (Sullivan et al, 2020, eTG, 2019). There is an unmet clinical need to address serious, common viral infections in Australia, now and into the future.
Clinical management
Current clinical management pathway is as below
Source: author-made, based on eTG, 2019.
Regulatory process
An in vitro ultraviolet blood irradiator by PIT Medical Systems GmbH is currently approved by the Australian Therapeutic Goods Administration (TGA #355414) which could be used for this purpose as well. The approval indicates the UVA PIT System can be used for Photo Immune Therapy on autologous blood, using UVA irradiation (in the presence of the photoactive substance 8-MOP) on extracorporeal circulating leukocyte enriched blood.
This submission is seeking to expand the use of the UV irradiation system in the existing MSAC 1651 – Integrated, closed-system, extracorporeal photopheresis systems for the treatment of chronic graft-versus-host disease, such that the technology may be used to treat influenza infections with government funding for this indication, to be considered as MSAC 1651.2.
Claimed benefits of the technology
While the majority of people who contract influenza have a mild disease process, a minority experience severe disease. Given the high incidence and seasonal nature of influenza infection, this minority with severe disease have a large public health impact. The mean annual number of confirmed cases of influenza between 2011-2018 was 95,624 (DOH, 2022). The latest hospital surveillance program estimated 10,000 admissions nationally per year with influenza (Cheng et al, 2013). Mortality varies by year, trending closely with vaccination rates and how well the vaccination matches circulating strains.
Severe disease is most commonly characterised by the development of viral pneumonia, but may additionally have gastrointestinal and neurological sequelae. Secondary bacterial infection may take advantage of depleted immune systems (Miethke et al, 2021).
Extracorporeal photopheresis has been used to date to bioengineer skin substitutes, aid wound healing, counteract chronic inflammation, treat burns and pressure ulcers, and improve postoperative healing (Kasmi et al., 2009, 234-42). Its safety is well documented, but the extent of its possible effective uses is yet underdeveloped.
In this utilisation, white blood cells are extracted via leukapheresis and treated with a photosensitizers before being irradiated with UVA light. The process can lead to activation of the parts of the immune system while moderating the pro-inflammatory cytokine storm which is implicated in the systemic inflammatory response (PIT Medical Systems 2018).
Thus, UVA irradiation for influenza treatment will fill a clinical need for an effective post-infection treatment for patients with influenza who are at risk of severe disease, or who already have severe disease requiring hospitalisation. It will provide a second line of protection when influenza vaccination doesn’t match circulating strains.
3. Evidence to support proposal for public funding
Oseltamivir: safety
Side effects of oseltamivir include nausea, vomiting, headache and pain and rarely, serious allergic reaction. The use in pregnancy, the elderly and those under 1 year of age hasn’t been well studied in terms of safety and efficacy. Oseltamivir has interactions with some immunosuppressant medications, and may be contraindicated (Product and Consumer Medicine Information Licence, 2022).
Antiviral resistance to Oseltamivir has been demonstrated, with particularly high risk of resistance in the immunocompromised, in children and when there is mixing of strains (antigenic shift) in outbreaks (Robson C et al., 2022).
Oseltamivir: efficacy
Oseltamivir shortens the duration of symptoms in uncomplicated influenza by approximately one day. There is evidence from low-level evidence trials to suggest oseltamivir reduces rates of pneumonia complications. No effect was found on the rate of hospital admissions (Robson C et al., 2022).
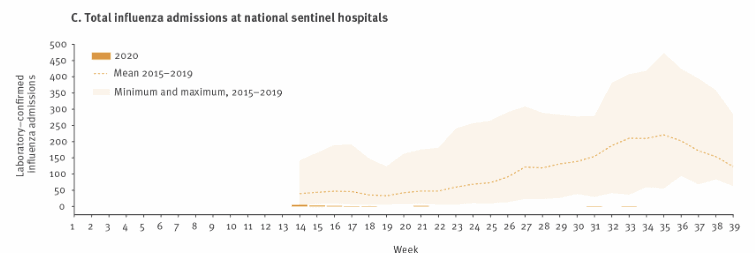
Effectiveness of the current treatment paradigm (vaccination +/- Oseltamivir) is monitored through the National Influenza Surveillance Scheme Reports. This graph demonstrates the wide range and seasonality of median hospital admission rate for laboratory confirmed influenza between 2015-2019.
Figure 1.Surveillance data on (C) total influenza admissions per week at national sentinel hospitals, Australia, as at 30 September 2020. (Sullivan et al, 2020)
ECP safety
The main risk of blood transfusion as a treatment category is patient misidentification leading to ABO incompatibility and immune reactions (Lifeblood, n.d.). The currently approved ECP system automates this process, to ensure that separation processing with methoxsalen and UV light and reinfusion are done without the potential for patient identification error to be introduced.
Blood transfusion requires haemodynamic stability, in order to tolerate the small volume loss, so unwell patients must be stabilised first with fluid resuscitation if they are hypotensive or have sepsis. Patients with severe heart failure are unlikely to tolerate the small volume shifts required for treatment, so will need to be assessed by a cardiologist on a case by case basis if their left ventricle ejection fraction is less than 25% (partial contraindication). ECP may be contraindicated in patients with severe coagulopathies, specialist advice is recommended (Mallinckrodt, 2016).
The system requires anticoagulation, typically with heparinized saline. People with adverse reactions to heparin, such as Heparin Induced Idiopathic Thrombocytopenia, will need specialist advice or close follow up .
The photosensitising agent methoxsalen is contraindicated in patients who are allergic to psoralens, those with a history of light-sensitive disease (such as patients with systemic lupus erythematosus with photosensitive disease, or those with aphakia). Severe hepatic or renal disease can affect the drug’s metabolism. It is not teratogenic, but shouldn’t be used in breastfeeding mothers (DermNet, 2007).
Systemic immune reaction (overactivation) may be minimised by the small volume of blood which is photosensitised (Hamblin, 2018, Kasmi et al., 2009, 234-42).
ECP effectiveness
Ultraviolet irradiation has demonstrated effectiveness to treat viral infection in a number of studies. The exact reduction in hospitalisations needs to be equated to present day hospitalisation rates.
Existing data describes 151 cases of acute bacterial infection in the blood (bacteraemia), with a 100% non-progression rate to septicaemia (Miley, 1942). A further study demonstrated 56% of presently septic patients recovered uneventfully following UV therapy (Miley, 1944). This limited data suggests this is an efficacious treatment for preventing the extreme inflammatory reactions which confer morbidity in systemic infection. This process has been validated by other studies (Miley, Seidel, Christensen, 1943, Barrett, 1940, Rebbeck 1942).
Surveillance presently includes sentinel general practice consultations for influenza-like illness, laboratory reports of influenza and absenteeism rates from a national employer, which is proposed to continue as monitoring for efficacy of treatment (DOH, 2022).
Dose and duration
The exact amount of UV A light applied to the blood product is adjusted depending on individual patient variables, particularly body weight. The dose of methoxsalen is standardised for adults and reduced in children (PBS, 2022).
As influenza does not remain in the body like many other viruses, a course of ECP on two consecutive days is usually sufficient to induce remission. In cases where symptoms are persisting after two weeks, another two consecutive days of ECP may be offered. Treatment may be performed as an inpatient for those hospitalised, or as an outpatient in those not requiring inpatient management but who are at high risk (Dermnet, 2007).
Treatment duration is 103 minutes for single lumen needle mode, 74 minutes for double lumen needle mode. It may be performed anywhere with a suitable Cellex machine, which is currently only in selected tertiary public and private hospitals (Photopheresis – PIT Medical Systems, 2018).
4. Approach to the economic evaluation
Financial comparison
Existing treatment
| Element | Cost |
| Oseltamivir | $40.99 |
| Total | $40.99 |
Existing treatment is a private script, meaning the cost is borne by the patient in its entirety.
Actual use of oseltamivir is only measured in hospitalised patients, where 57% received the medication (DOH, 2022). This cost is absorbed by Medicare or the private insurer.
Cost of treatment failure from existing treatments (i.e. requiring hospitalisation) has more wide ranging effects on the healthcare system. A public inpatient hospital stay per night in Australia in 2021 cost $2820.75 on average (AIHW, 2023). Average length of stay for influenza patients was 5 nights (DOH, 2022).
Proposed treatment
| Element | Cost |
| Methoxsalen | =$34.83 for one course (2 injections) |
| CELLEX® Photopheresis System operating costs, including staffing | = 1063.95 euro per procedure (Magaro et al, 2021) Approximately $1783.54 AUD |
| Total | $1818.87 |
The proposed technology would need funding from a government source, in this case the PBS and MBS, as the cost is prohibitive for an individual patient to fund it.
Economic evaluation
The time horizon applied will be a 3 year model, to allow for monitoring and evaluation as well as investigation of secondary outcomes of interest (influenza resistance surveillance). Primary outcomes will be demonstrated within a year and then averaged over a number of ‘flu seasons.
Discounting of DALYs at a rate of 5% will be applied as per MSAC standards. For complications of influenza, including heart attack, decompensated cardiac failure, pneumonia and death, standardised QALY will be applied to the proportionate rates of complication.
Productivity takes a hit during Influenza season, as surveys suggest between 1.4 and 2.7% of the workforce are absent due to Influenza-like-illness (DOH, 2022).
The perspective of the report is from the Australian Taxpayer. The Federal Government, by way of the Medical Benefits Schedule, will absorb the cost of the subsidy, but the perspective of this report is the overall costs to the economy and healthcare system.
Incremental cost effectiveness ratio
Hospitalisations are used here as a proxy for quality of life (QALY). It is documented that severe influenza, sepsis and Acute Respiratory Distress Syndrome all affect quality of life (Chen et al, 2017, Granja, C, 2004, Shah et al, 2021). The severity of each escalates in effect on QoL. Further analysis could be performed to identify ICER for hospitalised versus ICU patients.
Given oseltamivir reduces duration of symptoms by <24 hours and has no effect on hospitalisation, its effectiveness at preventing hospitalisation, or treating influenza, is close to zero.
Within the limitations of the underpowered studies demonstrating the use of ECP against infectious disease, estimations of ECP effectiveness at preventing hospitalisation in those at risk would be close to 100% given the favourable non-progression rate to severe disease. However, assuming the most conservative estimate of 56% (successfully treated in already already advanced disease given in Miles, 1942), the below ICER is produced for Year 1/
Year 1 ICER
= (Cost of technology 1/effectiveness of technology 1) / (cost of technology 2/effectiveness of technology 2)
= (141 709 046/ 0.1) / (139 938 107/ 0.56)
= 5.67
Budget Impact
This budget analysis excludes indirect benefits such as reducing secondary complications, reducing the number of antiviral resistant strains and increasing our responsiveness to epidemics and novel viruses.
Cost of treatment with oseltamivir can be estimated by present inpatient, outpatient use, plus the cost of hospitalisation via rate, length of stay and average cost of hospital stay.
Cost = 10 000 x 5 x 2820.75
= 141,037,500
Projected inpatient use of the technology would be those currently hospitalised with influenza (10,000 patients per annum). Outpatient use would be the proportion of incidence with risk factors (a 70% majority of laboratory cases will be <18 or >65 years old) (DOH, 2022).
Projected OP use = 95 624 x (0.7) x 1818.87
= 66,936.8
Projections note there will likely be an increase in the uptake of ECP as a preventative measure for those at risk is better understood by the clinician community, and a decrease in Oseltamivir over the course of the treatment being introduced. Appropriate usage will need to be reviewed frequently. Relevant risk factors will be adjusted based off novel clinical evidence.
Oseltamivir projection
| Year 1 | Year 2 | Year 3 | |
|---|---|---|---|
| Oseltamivir (inpatient use) | 10,000 x 0.57 x 44 = 250,800 | Previous year x 0.8 = 200640 | Previous year x 0.8 = 160512 |
| Oseltamivir (outpatient use) | 95,624 x 0.1 x 44 =420,745.6
| Previous year x 0.5 = 210372.8 | Previous year x 0.5 =105186.4 |
| Hospitalisations | 10,000 x 5 x 2820.75 = 141,037,500 | 5,000 x 5 x 2820.75 = 70,518,750 | 2,500 x 5 x 2820.75 = 35,259,375 |
| Total cost | =$141 709 046 | =$70 929 764.8 | =$35 525 073.4 |
ECP projection
| Year 1 | Year 2 | Year 3 | |
| ECP (inpatient use) | 10,000 x 1818.87 =18,188,700 | 5,000 x 1818.87 = 9,094,350 | 2,500 x 1818.87 = 4,547,175 |
| ECP (outpatient use) | 121,749,337.416 | 121,749,337.416 | 121,749,337.416 |
| Total cost | =$ 139 938 107 | = $130 843 687 | =126 296 512 |
Therefore, at present there is a total cost of $141,709,046 to the healthcare system from influenza and its current management. While Year 1 of therapy is projected to increase to $281,647,153, by year 3 this is expected to decrease to $161,821,585, not dissimilar to existing costing.
Likely utilisation of the technology or service
We recommend that a predicted versus actual utilisation monitoring process is implemented for the first 5 years of the service implementation. This will include the Drug Utilisation Sub-Committee monitoring methoxsalen usage, and the MBS Review Taskforce to monitor Australia-specific studies which will emerge in response to this addition to the management algorithm.
5. Relevant ethical, legal, social and implementation issues
Technological resources
Initial limitation of machines in large population centres is unlikely to significantly hinder implementation, as studies of viral transmission across Australia have consistently demonstrated major cities are the epicentres of previous influenza epidemics, thus they are the priority locations for antiviral measures to be expanded (Sullivan, 2020).
Equity
The disease does not affect people equally, leading to disproportionate burdens on the elderly (who may have inadequate seroconversion of vaccination), pregnant women and Aboriginal and Torres Strait Islander people. Surveillance suggests influenza rates were between 1.9 and 3.7 times higher among those identifying as Aboriginal and/or Torres Strait Islander (DOH, 2022).
Health system
It is important, also, to consider those 10,000 admissions in the context of a healthcare system which is often at capacity outside of influenza season, and doesn’t have surge capacity for this seasonal change. Improved treatment of Influenza will have flow on benefits to the healthcare system capacity.
Human resources
ECP requires trained nursing staff who are able to perform cannulation and supervising senior medical staff to confirm eligibility for treatment, lack of contraindications and to be on hand if adverse effects arise.
Potential future use
Influenza is the starting point, as it is the highest incidence notifiable disease in Australia, However, multiple diseases and pathogens can be addressed with this therapy, notably viruses which are likely to cause future epidemics and pandemics.
6. References
Australian Institute of Health and Welfare. (2023). Australia's hospitals at a glance . Retrieved from https://www.aihw.gov.au/reports/hospitals/australias-hospitals-at-a-glanc
Barrett HA (1940) The irradiation of auto transfused blood by ultraviolet spectral energy. Result of therapy in 110 cases. Med clin N Am 721(24):1040
Barrett HA (1943) Five years’ experience with hemo- irradiation according to the Knott technic. Am J Surg 61(1):42–53
Chen, J., Wu, J., Hao, S. et al. Long term outcomes in survivors of epidemic Influenza A (H7N9) virus infection. Sci Rep 7, 17275 (2017). https://doi.org/10.1038/s41598-017-17497-6
Cheng et al. (2013). Influenza epidemiology, vaccine coverage and vaccine effectiveness in sentinel Australian hospitals in 2012: the Influenza Complications Alert Network (FluCAN). Communicable Diseases Intelligence, 37(3), NA.
Consumer Medical Information - Tamiflu. (2022, October 2). medsinfo. http://medsinfo.com.au/consumer-information/document/Tamiflu_CMI
DOH. (2022, March 28). Communicable Diseases Intelligence 2021 - Report of the National Influenza Surveillance Scheme, 2011 to 2018. Department of Health and Aged Care. https://www1.health.gov.au/internet/main/publishing.nsf/Content/2A15CD097063EF40CA2587CE008354F1/$File/report_of_the_national_influenza_surveillance_scheme_2011_to_2018.pdf
Granja C. Quality of life of sepsis survivors: evaluation with a specific critical care questionnaire. Crit Care. 2004;8(Suppl 1):P345. doi: 10.1186/cc2812.
Hamblin MR. Ultraviolet Irradiation of Blood: "The Cure That Time Forgot"? Adv Exp Med Biol. 2017;996:295-309. doi: 10.1007/978-3-319-56017-5_25.
Infectious and communicable diseases. (2023, August 15). Australian Institute of Health and Welfare. https://www.aihw.gov.au/reports/australias-health/infectious-and-communicable-diseases
Kasmi, B., Inglefield, C., & Lewis, M. (2009). Autologous cell therapy: current treatments and future prospects. Wounds, 21(9), 234-42. PMID: 25903815.
Magarò A, Lucchetti B, Caime A, Lionetti MT, Laszlo D. Cost comparison of extracorporeal photopheresis technologies at the European Institute of Oncology. J Clin Apher. 2021 Jun;36(3):364-369. doi: 10.1002/jca.21870. Epub 2021 Jan 21. PMID: 33476456.
Mallinckrodt. (2016). For the listing of integrated, closed-system, extracorporeal photopheresis (ECP) systems. MSAC Application Number 1420. http://www.msac.gov.au/internet/msac/publishing.nsf/Content/6DDD8F69590FB97DCA25801000123C26/$File/1420%20-%20CTCL%20-%20Final%20Protocol%20v2.pdf
McKimm-Breschkin, J. (2013, June 3). H1N1, H5N1, H7N9? What on earth does it all mean. The Conversation. https://theconversation.com/h1n1-h5n1-h7n9-what-on-earth-does-it-all-mean-14815
Miley, G. 1944. Efficacy of ultraviolet blood irradiation therapy in the control of staphylococcemias. American Journal of Surgery, 64: 3:313-322, https://doi.org/10.1016/S0002-9610(44)90499-X.
Miley GP, Seidel RE, Christensen JA (1943) Preliminary report of results observed in eighty cases of intractable bronchial asthma. Arch Phys Ther 533(24
Miethke, M., Pieroni, M., Weber, T. et al. Towards the sustainable discovery and development of new antibiotics. Nat Rev Chem 5, 726–749 (2021). https://doi.org/10.1038/s41570-021-00313-
Oseltamivir, capsules 30 mg, 45 mg and 75 mg, powder for oral suspension, 12 mg per mL, Tamiflu®, November 2008. (2009, March 19). PBS. https://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/2008-11/pbac-psd-oseltamivir-nov08
PBS. (2022, October 2). METHOXSALEN. Pharmaceutical Benefits Scheme. https://www.pbs.gov.au/medicine/item/12154Q-12156T-12162D-12173Q-12839R-12854M-12855N-12876Q
Peacock, Dehle, Zapata, Prince, Gennari, Taylor. 2022. Cost-Effectiveness of Extracorporeal Photopheresis for the Treatment of Patients With Erythrodermic (Stage T4, M0) Cutaneous T-Cell Lymphoma in the Australian Setting. Value in Health. 25, 6: 965-974. https://doi.org/10.1016/j.jval.2021.11.1364.
Photopheresis – PIT Medical Systems. (2018). PIT Medical Systems. https://pitsystem.eu/photopheresis/
Phototherapy. UVA photo(chemo)therapy. (2007). DermNet. https://dermnetnz.org/cme/phototherapy/uva-photochemotherapy
Product and Consumer Medicine Information Licence. (2022). TGA eBS. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2020-CMI-02676-1&d=20231102172310101&d=20231112172310101
Rebbeck EW (1942) Double septicemia following prostatectomy treated by the knott technic of ultraviolet blood irradiation. Am J Surg 57(3):536–538
Robson C, Baskar SR, Booy R, Ferguson PE, Gilroy N, Kok J, et al., .. (2022, April). Influenza: overview on prevention and therapy. Australian Prescriber, 42(42), 51-55. https://doi.org/10.18773/austprescr.2019.013
Shah R, Ali FM, Nixon SJ, et alMeasuring the impact of COVID-19 on the quality of life of the survivors, partners and family members: a cross-sectional international online surveyBMJ Open 2021;11:e047680. doi: 10.1136/bmjopen-2020-047680
Sullivan, e. a. (2020). Where has all the influenza gone? The impact of COVID-19 on the circulation of influenza and other respiratory viruses. Euro Surveill, 25(47), 2. The impact of COVID-19 on the circulation of influenza and other respiratory vihttps://doi.org/10.2807/1560-7917.ES.2020.25.47.2001847
Therapeutic Guidelines, e. (2022, October 2). .Influenza. eTG. Retrieved November 2, 2023, from https://tgldcdp.tg.org.au.acs.hcn.com.au/viewTopic?etgAccess=true&guidelinePage=Antibiotic&topicfile=influenza&guidelinename=Antibiotic§ionId=toc_d1e63#toc_d1e63
Transfusion risks. (n.d.). Lifeblood. Retrieved November 13, 2023, from https://www.lifeblood.com.au/patients/receiving-a-transfusion/informed-consent/transfusion-risks
Figure 1
Sullivan Sheena G, Carlson Sandra, Cheng Allen C, Chilver Monique BN, Dwyer Dominic E, Irwin Melissa, Kok Jen, Macartney Kristine, MacLachlan Jennifer, Minney-Smith Cara, Smith David, Stocks Nigel, Taylor Janette, Barr Ian G. Where has all the influenza gone? The impact of COVID-19 on the circulation of influenza and other respiratory viruses, Australia, March to September 2020. Euro Surveill. 2020;25(47):pii=2001847.